A novel modality for selectively inactivating homomeric proteins, common in disease-driving pathways, by inducing their polymerization. PINCH compounds are dimeric small molecules that bind homomeric proteins and trigger their assembly into insoluble aggregates, blocking function without relying on inhibition or degradation through cellular accessory proteins (like E3 ligases for PROTACs).
- Functional inactivation of disease-driving homomeric proteins (e.g., transcription factors, metabolic enzymes, scaffold-like proteins etc.)
- Potential treatments for cancers, chronic kidney disease, and metabolic disorders
- Alternative to PROTACs or inhibitors when degradation or inhibition is ineffective or undesirable
- Hetero-PINCHs link two homomeric targets (e.g., Keap1, BCL6) for co-polymerization and dual downregulation, enabling synthetic lethality approaches
- High selectivity and generalizability
- Targeted aggregation - Acts only on homomeric proteins with defined binding motifs
- Extended duration of action
- Effective in proteasome-independent settings
- Possibility to inactivate ‘bystander’ proteins
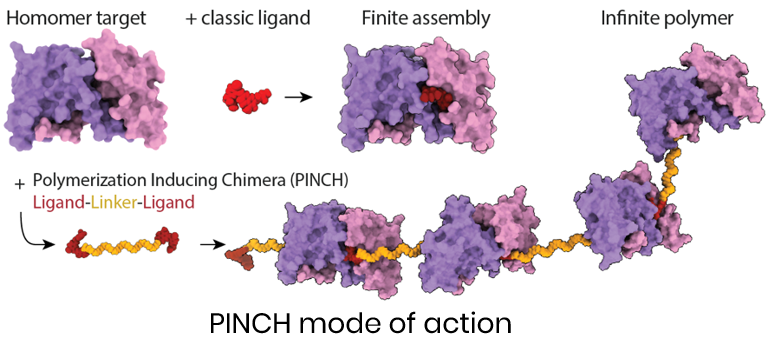
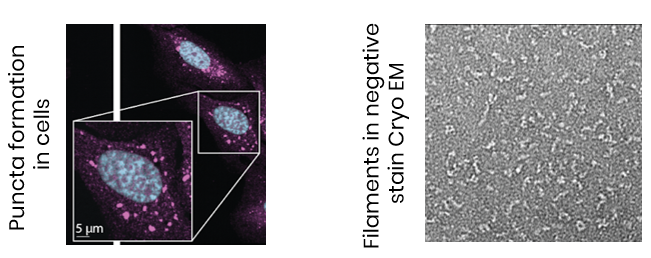
PINCH compounds targeting clinically relevant proteins like BCL6, and Keap1, have shown nanomolar potency and durable activity in vitro and in cells, with validated polymerization in cells, and differentiated functional effects.
Livnah et al, bioRxiv, 2025


