6026
Overview
LOX is a key driver of fibrosis and tumor progression through its role in extracellular matrix remodeling. We developed a set of LOX inhibitors based on LOX’s natural prodomain (LPD) to treat fibrosis, Duchenne Muscular Dystrophy (DMD), and cancer.
Applications
- Treatment of fibrotic diseases and metastatic cancer
- Mutation-independent treatment of DMD
Differentiation
- Selective: targets only extracellular LOX
- Broad: applicable across a range of fibrotic diseases and cancer
- Validated: in vivo efficacy in fibrosis and cancer mouse models
- Stable: high-affinity Fc-fusion inhibitor
- Antibody-like: durable and selective design
Development Stage
- Fc- protein inhibitor purified, characterized for stability and high-affinity binding
- Showed significant reduction in fibrosis and improved muscle function in DMD mice
- Reduced lung metastases in a melanoma mouse model
LOX inhibitor
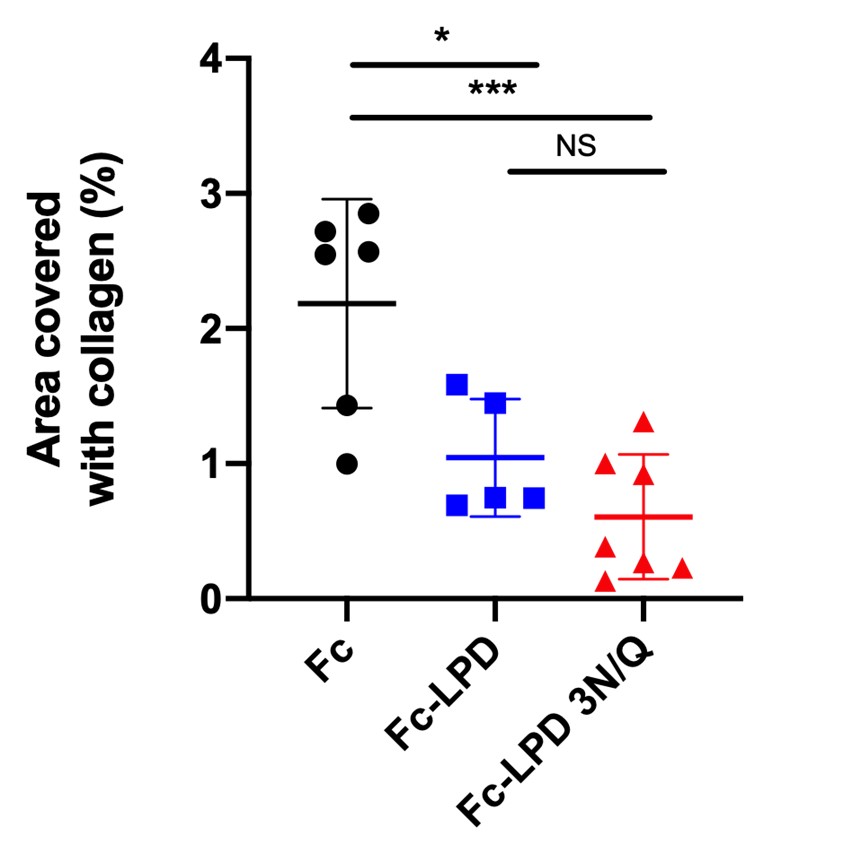
LOX inhibitors (LPD) reduced collagen deposits in the MDX Duchenne mouse model
Patent Status:
USA Published: Publication Number: 20240398906 USA Published: Publication Number: 2022-0049236-A1
Contact for more information


