A novel method to treat tyrosine kinase inhibitor (TKI) resistant cancer by using a combination of three antibodies that target EGFR (ERBB1), HER2 (ERBB2), and HER3 (ERBB3) receptors.
The ErbB/HER family of receptor tyrosine kinases includes epidermal growth factor receptor (EGFR, also termed ErbB-1, HER1), HER2 (ErbB-2), HER3 (ErbB-3) and HER4 (ErbB-4). The ErbB/HER family members and their multiple ligand molecules form a layered signaling network, which is implicated in several human cancers. ErbB activation leads to downstream stimulation of several signaling cascades that influence cell proliferation, angiogenesis, invasion, and metastasis. Because of their oncogenic potential and accessibility, ErbB/HER proteins have emerged as attractive targets for pharmaceutical interventions, mainly tyrosine kinase inhibitors (TKIs) and anti-HER monoclonal antibodies (mABs). However, while many cancer patients were found to be sensitive to ErbB-targeted therapy, many other patients are resistant to treatment, and even among the initially responsive patients a large percentage experience tumor recurrence and become resistant to therapy. Thus, for example, despite initial dramatic response of non-small cell lung cancer (NSCLC) patients to TKIs, all patients acquire resistance within approximately one year, commonly due to malignant cells acquiring resistance through new mutations in receptors that block the effects of TKIs. Therefore, there is a need for an effective treatment against TKI resistant lung cancer.
The team of Prof. Yosef Yarden have discovered that using a triple mAb combination targeting three HER family receptors (EGFR, HER1, and HER2) prevented the activation of downstream kinase cascades, accelerated the degradation of the receptors, and inhibited the proliferation of tumor cells but not of normal cells. This treatment can be used to treat AKI - resistant tumors as well as augment TKI dependent therapies.
The team of Prof. Yarden discovered feedback regulatory loops that increase the abundance of HER2 and HER3, and activate another receptor tyrosine kinase, MET, in response to antibody-induced blockade of EGFR. The team found that using mAbs inhibiting HER2 and HER3 along with EGFR, could effectively abrogate the compensatory mechanism and inhibit NSCLC cell growth.
- A novel treatment for TKI resistant tumors.
- A feasible pharmacological option for treating numerous lung cancer patients who will inevitably develop resistance to the currently applied inhibitors of EGFR.
- In contrast to TKIs, mAbs offer target destruction, high specificity, and the ability to cooperate with chemotherapy.
- mAbs targeting ErbB/HER receptors already exist with clinical approval.
The research team tested the concept on two TKI resistant NSCLC cell lines, H1975 and PC9ER, using different combinations of anti-EGFR (1), HER2 (2), and HER3 (3) antibodies. The tests also used a combination of the TKI erlotinib (Erlo) along with mAbs against HER2 and HER3 for comparison. Both TKI resistant cell lines tested had the greatest inhibitory effect when using a of three antibodies targeting different receptors simultaneously.
Additionally, the triple mAb combination treatment was tested in animal models comprising xenografts of NSCLC cells (H1975) grown in mice. Figure 1 exhibits that only the combination of antibodies targeting all three receptor tyrosine kinases (EGFR, HER2, and HER3) inhibited tumor progression.
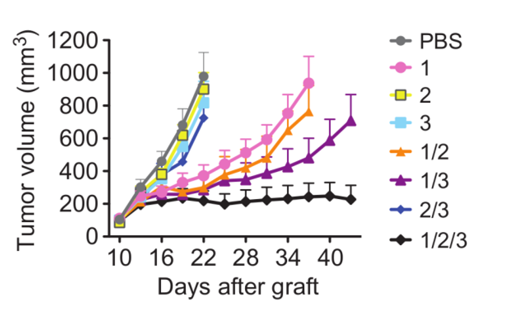
Figure 1: Tumor growth of xenografted H1975 cells in CD1-nu/nu mice. Each mouse was treated every three days with either a single, double, or triple mAb treatment of (1) EGFR, (2) HER2 , or/and (3) HER3. Image modified from Mancini et al. Sci. Signal. 2015.


