A novel therapeutic strategy for Amyotrophic Lateral Sclerosis (ALS) targets impaired microRNA (miRNA) biogenesis—a key molecular defect found in both sporadic and familial ALS. This approach leverages Enoxacin, an existing fluoroquinolone drug, to enhance global miRNA production and restore cellular regulation disrupted in motor neurons. This repurposing strategy offers a promising, low-risk path to treating ALS by targeting a previously unaddressed mechanism of disease progression.
- Treatment of ALS
- Repurposes an FDA-approved drug, reducing development time and regulatory hurdles
- Targets a novel disease mechanism: impaired miRNA biogenesis
- Demonstrated functional improvement in two ALS mouse models
- Early clinical trial results support safety and potential therapeutic effect
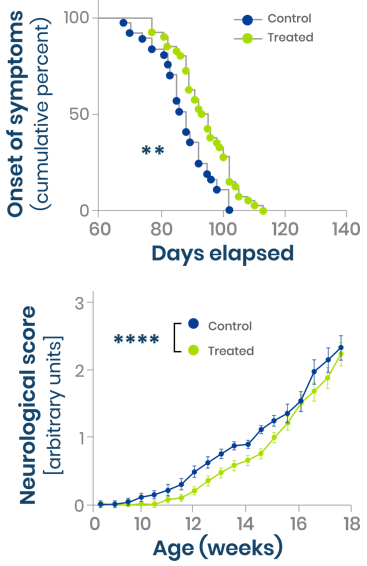
Beneficial impact of Enoxacin on neuromuscular function of SOD1 G93A mouse ALS model
Phase 1b/2a clinical trial (REALS) completed in ALS patients demonstrating safety, pharmacokinetics, and exploratory target engagement.


