Proteasome-derived defense peptides (PDDPs) represent a novel class of antimicrobial peptides (AMPs) generated through proteasomal degradation. These peptides disrupt bacterial membranes and provide a cell-autonomous defense mechanism against infections. By leveraging in silico prediction and mass spectrometry analysis, researchers have identified over 270,000 putative PDDPs, with several validated for antimicrobial efficacy in vitro and in vivo.
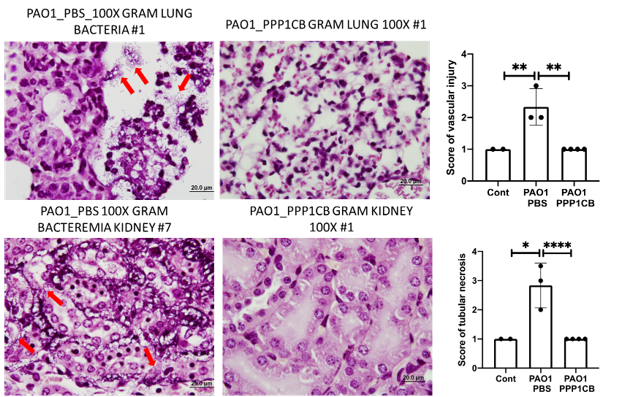
- Sepsis treatment – PDDPs have shown efficacy in murine models of Pseudomonas-induced bacteremia (including sepsis) and pneumonia , reducing bacterial load and tissue damage.
- Broad-spectrum antimicrobial therapy – Effective against multidrug-resistant pathogens such as Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa.
- Biofilm disruption – PDDPs can prevent and degrade bacterial biofilms, a key factor in persistent infections
- Anti-bacterial coatings – PDDPs could be used to protect different organisms, utilized in biotechnology applications (e.g. surfaces) and other products from bacterial spoilage, e.g., food preservation
- Endogenous origin – Reduced risk of immune activation compared to synthetic AMPs.
- Short – The average length of the peptides is around 17 AA. Reduced production costs and imprived.
- In vivo stability – Demonstrated stability against proteases and long-lasting efficacy.
- Broad-spectrum activity – Effective against both Gram-positive and Gram-negative bacteria.
- Minimal resistance risk – Targets bacterial membranes, reducing resistance development.
- Potential for combination therapy – Synergistic effects with existing antibiotics or other AMPs.
- Potential targeted drug delivery – Controlled AMP release at infection sites may be achieved using protease-cleavable di-/tri-peptide constructs.
PDDPs have been validated in vitro for antimicrobial activity against multiple pathogens and in vivo in murine models of bacteremia and sepsis, demonstrating significant bacterial clearance and tissue protection.
Ongoing preclinical studies focus on optimizing peptide stability, bioavailability, and formulation, including protease-cleavable constructs and combination therapies. Future steps include expanding efficacy testing in larger animal models and evaluating clinical translation potential.
Goldberg K et al. Nature. 2025


