Bacterial and plant immune systems rely on Toll/Interleukin-1 receptor (TIR) domains to detect pathogens and initiate defense responses through immune signals comprising isomers of cyclic ADP-ribose. Weizmann researchers discovered viral proteins that bind and neutralizes these immune signals, effectively suppressing TIR-mediated immunity. These insights establish cADPR isomers as universal immune messengers and position the viral proteins discovered at the Weizmann as valuable tools for developing phage-based therapeutics, immune modulators, and technologies that strengthen agricultural resilience.
- Phage therapy: Engineering “super-phages” expressing Tad1 genes to overcome bacterial immune defenses.
- Agriculture: Modulating plant TIR immunity via cADPR isomers to boost disease resistance or prevent overactivation.
- Drug discovery: Design of synthetic cADPR isomer analogs or inhibitors to regulate TIR-based innate immunity.
- Synthetic biology: Development of programmable signaling circuits based on immune molecule production or sequestration.
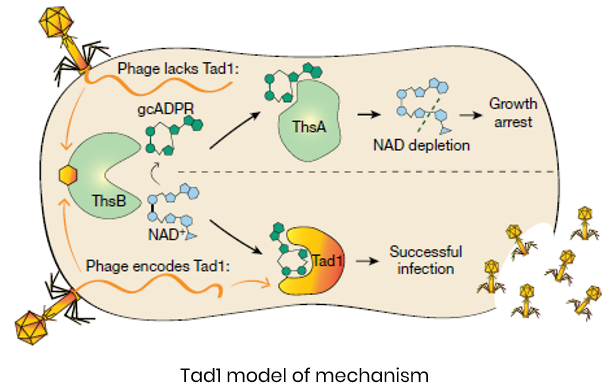
- Cross-kingdom relevance: The same messenger drives bacterial and plant immunity
- Unique mechanism: Viral proteins act as molecular “sponges,” binding and neutralizing immune messengers
- Structural insight: Crystal structures reveal immune messenger –protein interactions for rational inhibitor design
Proof of concept demonstrated: Viral anti-defense proteins were validated across multiple phages; immune signaling molecule identified and structurally resolved (1.9–2.8 Å); viral sponges were shown to inhibit bacterial and plant TIR signaling in cells and in vitro.


