A novel combination therapy pairing ketamine with retigabine, an FDA-approved KCNQ channel activator, offers a promising strategy for mood-related disorders.
By enhancing ketamine’s efficacy via KCNQ activation, this approach may benefit patients unresponsive to standard treatments, while enabling lower dosing, improving safety, and reducing adverse effects such as dissociation and addiction risk.
- Potential to expand treatment opportunities for patients with treatment-resistant depression (TRD) unresponsive to existing therapies
- Safer long-term antidepressant use: Enables lower ketamine doses, reducing the risk of addiction, dissociation, and other adverse effects
- Sustained antidepressant effect
- Improved safety – minimizes dose-related side effects
- FDA-approved drugs, simplifying clinical adoption
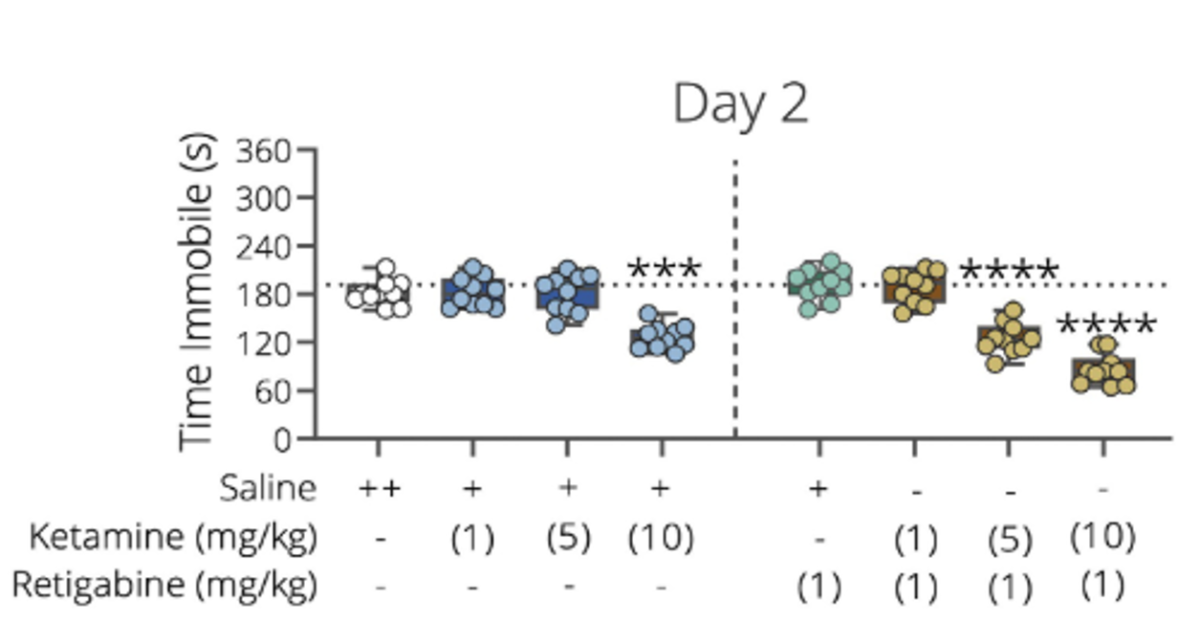
A combination of ketamine and retigabine improves mice results in the forced swim test (FST) after two days
The combination has been validated in preclinical studies using ex vivo electrophysiology and in vivo behavioral models. Ketamine and retigabine co-administration significantly enhanced antidepressant-like effects in mice, including reduced immobility in the forced swim test, even at sub-effective ketamine doses, supporting improved safety and efficacy.


