A method for controlling gene expression using a novel phage-based transcription factor-peptide system.
A fundamental challenge of synthetic biology and its clinical applications is the capability to control gene expression. Presently, gene expression systems for research and industry have several limitations, such as a small number of available systems, inconsistent behavior among systems, lack of sufficient dynamic range of expression, and the requirement for high concentrations of a signaling molecule to induce/repress a gene. Consequently, there is a strong need for a better and diverse array of gene expression systems.
The team of Prof. Sorek at the Weizmann Institute of Science (WIS) has made the paradigm – shifting discovery that bacteriophages have a quorum sensing system, similar to that found in bacteria. The novel system, named Arbitrium by the Sorek team, communicates to said phages residing within bacteria, whether a lytic or lysogenic cycle is to be entered. Arbitrium system can be harnessed to a wide range of synthetic-biology and biotechnological applications.
Phages can infect bacteria either in a lytic cycle, which lyses the bacterial wall, or in a lysogenic cycle in which the phage genome integrates into the bacterial genome. The research team of Prof. Sorek discovered the novel Arbitrium communication system that enables lytic or lysogenic cycle (Fig. A). The Arbitrium system functions on the basis of three genes, aimR, aimP, and aimX. AimR is a dimeric receptor that binds and activates the transcription of gene aimX, whereby AimX inhibits lysogeny leading to lysis (Fig. B). AimP is a six amino acid pro-peptide (arbitrium peptide) that is expressed following infection, secreted extracellularly, and post-translationally modified. After the arbitrium peptide accumulates to a specific concentration, the arbitrium peptide is internalized and bound by the AimR receptor, which inhibits the expression of AimX and leads to lysogeny (Fig. C). The Sorek team further determined that when infected bacteria are incubated with the arbitrium peptide from the same phage that infected them, the bacterial growth curve changes due to a shift to a lysogenic cycle (Fig. D). However, when incubated with an arbitrium peptide from a different phage, the bacterial growth curve does not shift, regardless of peptide concentration (Fig. E).
- Unique system – Effectively induces or shuts off expression of a gene.
- Standard Chemistry – Uses regular six amino acid peptides, therefore standard synthesis can be used.
- Wide Dynamic Range
- Over 100 Different Systems
- Uniformity Among Systems
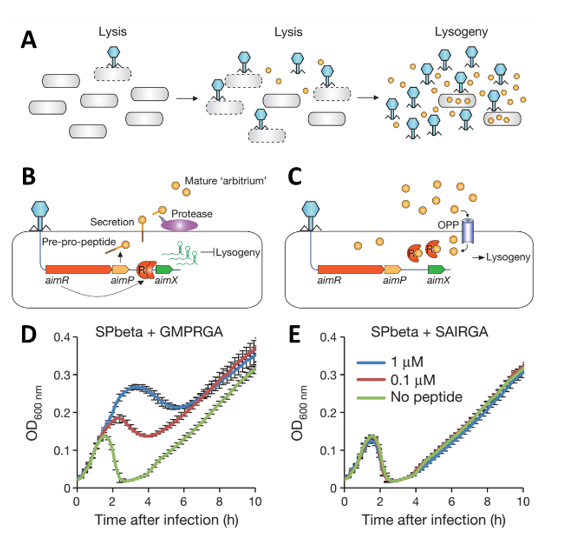
Schematic of arbitrium peptide accumulation during phage infection of bacteria. B) Post-infection aimR and aimP expression leading to lysis and AimP accumulation. C) At later stages after infection, lysogeny is preferred due to inhibition of AimX expression. D-E) Growth curves of B. subtillis in the presence of Phage SP Beta with either its corresponding arbitrium peptide (D) or arbitrium peptide from the phi3T phage (E). (Image modified from Erez Z, et al. Nature. 2017 Jan 26;541(7638):488-493. doi: 10.1038/nature21049. Epub 2017 Jan 18)
Erez, Zohar, Ida Steinberger-Levy, Maya Shamir, Shany Doron, Avigail Stokar-Avihail, Yoav Peleg, Sarah Melamed, et al. 2017. “Communication between Viruses Guides Lysis-Lysogeny Decisions.” Nature 541 (7638): 488–93.


