ALS is a severe neurodegenerative disorder with no cure and highly variable disease progression, which complicates clinical trial design and therapeutic development. This technology offers a novel, minimally invasive prognostic tool that measures circulating levels of miR-181—a brain- and spinal cord-enriched microRNA—to stratify patients by disease severity. When combined with NfL, it provides a powerful RNA–protein biomarker pair to enhance clinical trial precision and reduce variability.
- Prognostic tool – Predicts disease progression in ALS patients
- Clinical trial optimization by patient stratification – Enables a more precise and balanced grouping of patients in clinical trials
- Therapeutic monitoring – Assesses response to ALS treatments as a pharmacodynamic biomarker
- Minimally invasive, blood-based biomarker
- Improves accuracy of ALS prognosis
- Validated in two large patient cohorts
The biomarker has been validated in large, independent ALS patient cohorts using next-generation sequencing. Its prognostic value, alone and in combination with NfL, has been robustly demonstrated, positioning it at an advanced research stage with strong potential for clinical validation.
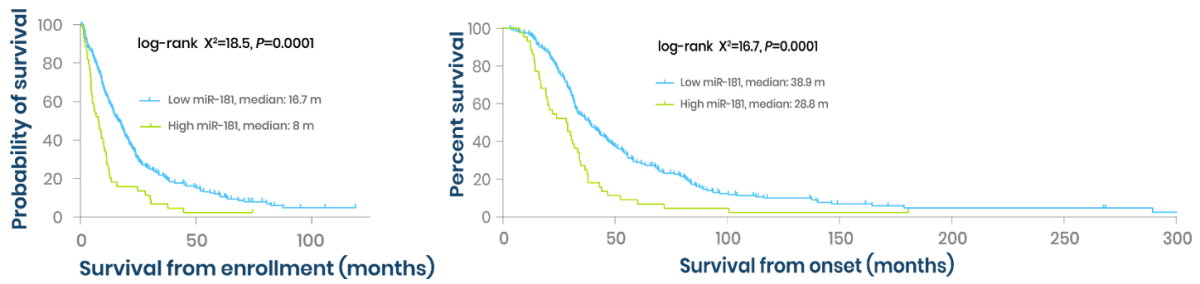
miR-181 is a prognostic biomarker of ALS - Kaplan–Meier curves on 248 ALS patients: 204 patients with subthreshold (light blue) versus 44 patients with suprathreshold (green) miR-181 levels from enrollment or onset.
Magen et al., Nat Neurosci, 2021. https://doi:10.1038/s41593-021-00936-z
Benatar et al., eBioMedicine, 2024. https://doi.org/10.1016/j.ebiom.2024.105323


