Malaria, caused by Plasmodium falciparum, remains a leading global health burden with growing drug resistance threatening current therapies. This technology introduces small-molecule inhibitors (i14G1s) that disrupt the interaction between translation initiation factors eIF1 and eIF4G1, a critical step in mRNA translation. Short-term treatment of P. falciparum with i14G1s was shown to inhibit parasite growth and block translation initiation, highlighting a novel therapeutic strategy that exploits the parasite’s translational machinery.
Malaria, caused by Plasmodium falciparum, remains a leading global health burden with growing drug resistance threatening current therapies. This technology introduces small-molecule inhibitors (i14G1s) that disrupt the interaction between translation initiation factors eIF1 and eIF4G1, a critical step in mRNA translation. Short-term treatment of P. falciparum with i14G1s was shown to inhibit parasite growth and block translation initiation, highlighting a novel therapeutic strategy that exploits the parasite’s translational machinery.
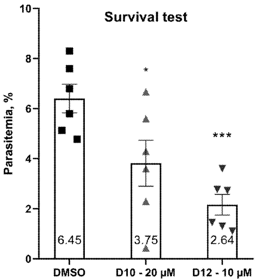
P. falciparum treated with i14G1-10 (20 µM) or i14G1-12 (10 µM) showed 42% and 69% reduced parasitemia after 24 h.
- Novel mechanism: first in-class inhibitors of eIF1–eIF4G1 interaction
- Direct parasite targeting: blocks growth and translation
- Resistance bypass: acts on a non-traditional pathway
- Screening platform: HTS-identified, enabling optimization
Small-molecule inhibitors (i14G1s) were identified through screening of 100,000 compounds and validated to specifically disrupt eIF1–eIF4G1 interactions.
Mechanistic studies confirm inhibition of translation initiation in human cells, and short-term treatment of P. falciparum demonstrated suppression of parasite growth.


