Dysfunctional immune circuits underlie many diseases, including cancer and autoimmunity. Current immunotherapies often target a single pathway or cell type, limiting their efficacy. We built a powerful proprietary discovery platform, based on AI-driven multiomics analysis of human clinical samples, to map and reprogram dysfunctional immune circuits. This approach enables the engineering of bispecific immune cell engagers (BiCEs), a revolutionary drug platform that engages physical interactions between two types of immune cells to reignite functional immune networks.
Our lead BiCE bridges PD-1+ T cells and Clec9A+ type 1 conventional dendritic cells (cDC1) to activate a critical cDC1–T cell interaction essential for potent anti-tumor activity. BiCE induces remarkable efficacy in anti-PD-1 unresponsive tumors, through unique MoA compared to traditional immune check point inhibitors.
- Treatment of anti-PD-1 refractory solid tumors, including breast, colorectal, lung, and melanoma cancers
- Treatment of autoimmune diseases, including multiple sclerosis and rheumatoid arthritis
- Top-down approach guided by deep unbiased data analysis of clinical samples
- Rational targeting of immune circuits to enable therapeutic immune modulation across diverse indications
- Immune activation in cancer or suppression in autoimmunity, depending on the circuit targeting
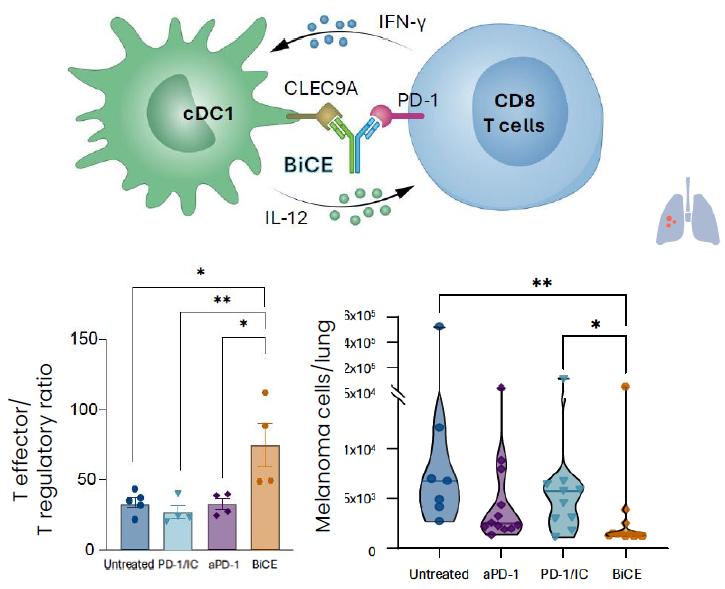
PD-1/CLEC9A BiCE restores immune cell balance to support anti-tumor activity, superior to aPD-1,across multiple aggressive cancer types
PD-1/CLEC9A BiCE induced immune reprogramming and potent anti-tumor activity in mouse models
PD-1/CLEC9A pathways validated in human tumors
Suppressive BiCEs, based on human validated targets, show immune reprogramming and efficacy in rheumatoid arthritis and multiple sclerosis mouse models



