Malaria remains a major global health challenge, with increasing resistance to current drugs and limited treatment options that selectively target infected red blood cells. This technology introduces synthetic antimicrobial peptides (AMPs) composed of leucine and lysine in D- and L- configurations. These peptides selectively penetrate and disrupt Plasmodium falciparum-infected red blood cells (Pf-iRBCs) without affecting healthy cells, offering a highly specific, non-toxic, and resistance-resilient therapeutic approach.
Applications
- Standalone treatment for malaria caused by Plasmodium falciparum
- Combination therapy with existing antimalarial drugs (e.g., artemisinin, chloroquine)
- Reduces transmission by targeting the P. falciparum membrane via a non-receptor mechanism with low resistance risk
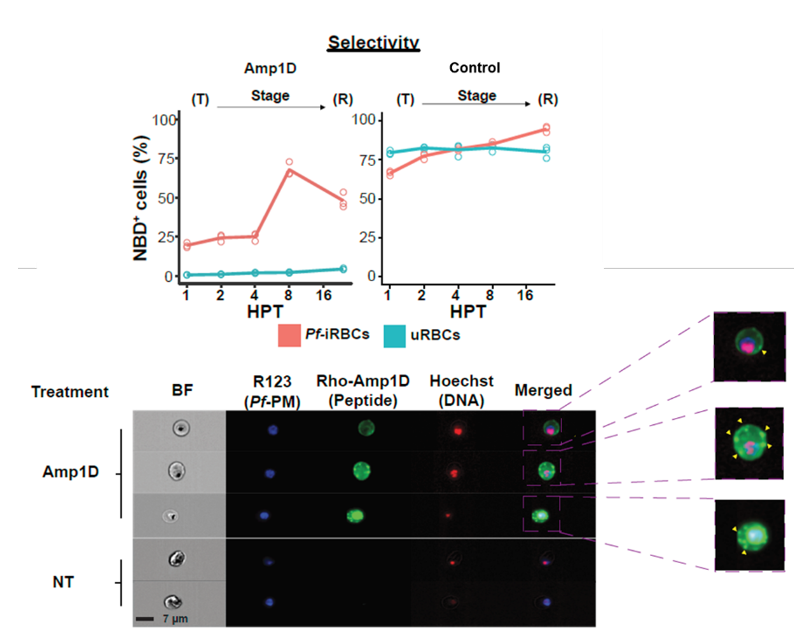
- Selectively targets infected red blood cells while sparing naïve RBCs
- Minimal hemolysis, with a promising IC50 in unpublished results
- Cholesterol-dependent, non-receptor-mediated mechanism ensures high specificity for Pf-iRBCs and lowers the risk of resistance development
- Compatible with drug conjugation for targeted delivery.
Demonstrated potent in vitro activity against P.falciparum with clear selectivity and a favorable safety profile. Mechanism of action confirmed via flow cytometry, AFM, and biophysical assays. The initial IP was filed; further validation is underway for clinical translation.
Kiper E, Ben Hur D, et al. J Biol Chem. 2025;301(4):108298.
Anti-Pf AMPs selectively bind to Pf-iRBCs, with maximal binding appearing at the late trophozoite–schizont stage, 8 Hours post-treatment.