17039
Overview
A novel approach for enhancing the efficacy of PD-L1 targeting antibodies by harnessing beneficial FcγR signaling pathways.
Strategy involves either
(1) Co-administration of PD-L1 targeting antibodies with an FcγRIIB-blocking antibody or
(2) Glycoengineering of the Fc region of PD-L1 targeting antibodies, such as Avelumab, to increase their affinity for activating Fcγ receptors
This approach significantly improves immune activation and tumor response to treatment.
Applications
- Enhanced anti-PD-L1 therapy for cancer treatment
- Potential applications in autoimmune and inflammatory diseases
Differentiation
- Glycoengineering of PDL-1 antibody to harness beneficial FcγR pathways
- Improved immune system activation for better and persistent tumor clearance
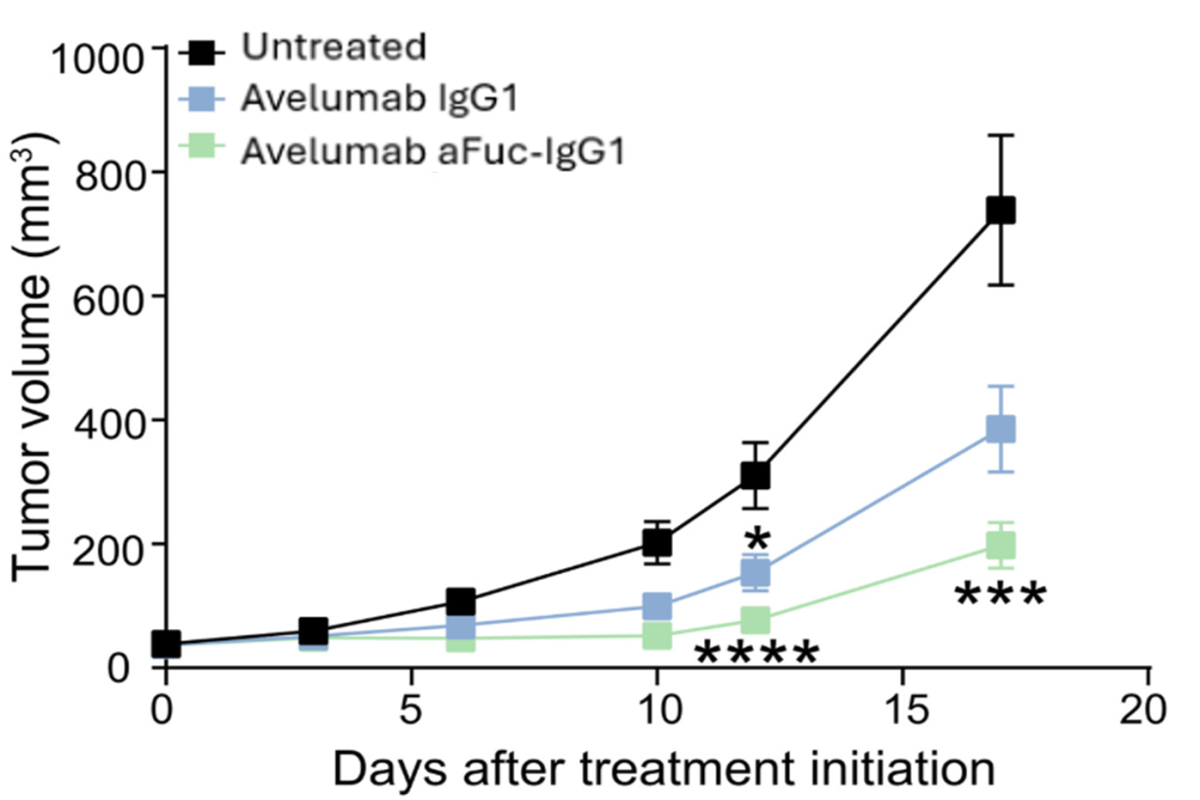
Fc glycoengineered PD-L1 antibody is showing improved anti-tumor activity in mice bearing MC38 colorectal tumors
Development Stage
- Validated in preclinical models, including mice with MC38 colorectal tumors and and mice with B16-F10 melanoma
- Fully humane Ab available for clinical evaluation
References
Cohen Saban et al. Sci. Immunol. 8, eadd8005 (2023).
Patent Status:
USA Published: Publication Number: 2023-0063965-A1
Contact for more information


