Accurate detection of minimal residual disease (MRD) is critical for predicting relapse in hematological cancers. However, current diagnostic tools often fail when therapeutic antibodies mask target markers, leading to false negatives. The B-probe technology addresses this unmet need by enabling sensitive and specific detection of masked cell-surface biomarkers. By tagging fluorescently labeled antibodies or binders to a bacterial cell, B-probe forms an avidity-based probe with exceptionally high sensitivity, specificity, modularity, and fluorescent intensity—overcoming the limitations of standard antibody-based assays.
- Detection of MRD markers in blood samples from multiple myeloma and leukemia patients
- Identification of cell-surface markers masked by immunotherapies (e.g., CD3, CD20, CD38)
- Detect low abundant cells within diverse cell population with flow cytometry
- Research-use applications for tracking immunotherapy response
- Platform for multiplexed cell-surface biomarker analysis
- High sensitivity and specificity
- Strong fluorescence signal via multivalent bacterial conjugates
- Modular, adaptable to new targets
- Stable, self-replicating bacterial core enables scalable probe production
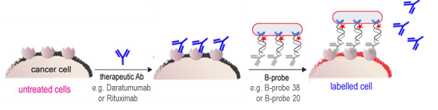
Proof-of-concept demonstrated in CD3 model systems using FACS analysis. B-probes showed 90% recovery of labeled cells on antibody-saturated targets and successfully detected 0.01% spiked cells undetectable by conventional antibodies. Lab pipeline established for adding new binders and targets. Next steps include expanding target range (e.g., CD20, CD38), adapting to clinical sample types and improving signal amplification.

Dr. Vered Pardo Yissar

