The growing use of lithium iron phosphate (LFP) batteries creates a need for efficient recycling. Unlike Li-Co-Ni batteries, LFPs yield only lithium as a valuable metal, making acid-based methods costly and inefficient. This technology applies a scalable pyrometallurgical process: aluminum is removed with sodium carbonate, lithium carbonate is recovered via cold-water leaching after sintering, and remaining solids are smelted to produce aluminum oxide and copper ingots—without hazardous chemicals or waste.
- Recycling of spent LFP batteries from electric vehicles and stationary energy storage systems
- Production of battery-grade lithium carbonate for reuse in cathode manufacturing
- Recovery of high-purity aluminum oxide and metallic copper as industrial feedstocks
- Integration into large-scale battery recycling facilities for sustainable material supply chains
- No hazardous chemicals: avoids strong acids, corrosive agents, and costly reagents
- Multi-metal recovery – produces lithium carbonate, aluminum oxide, and copper ingots
- Environmentally sustainable – no hazardous liquid or solid waste generated
- Industry-ready process – based on established, scalable industrial operations
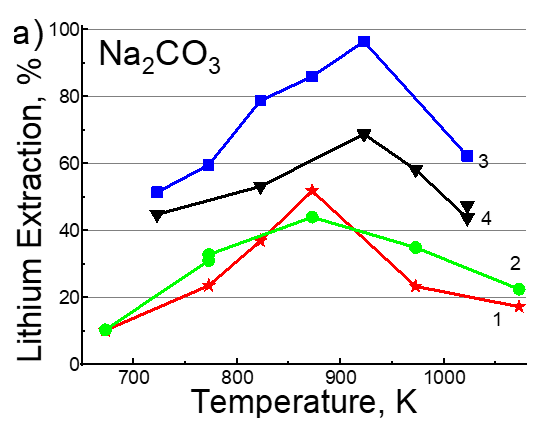
Lithium carbonate extraction yield after ice-water leaching with Me₂CO₃ excess: (1) 10% air, (2) 30% air, (3) 10% CO₂, (4) 30% CO₂.
Laboratory validation completed with both pure LiFePO₄ and real spent LFP batteries, achieving lithium recovery yields of 90–100% as lithium carbonate. Copper and aluminum recovered in high-purity solid forms. Next step: scale-up to a mini pilot unit (processing several kilograms of spent LFP batteries) within ~1 year, pending resources.

Dr. Vered Pardo Yissar

