18592
Overview
Aerobic oxidation is a key industrial process for producing valuable chemicals from alkenes and alkanes, abundant in petroleum and natural gas. However, existing methods are inefficient and unsustainable. This technology offers a novel, sustainable approach for aerobic electrochemical oxidation of hydrocarbons, utilizing copper-tungsten oxide catalysts to achieve efficient and selective transformations.
Applications
- Oxidation of ethane to Ethanol and /or Acetic Acid
- Electrocatalytic aerobic oxidation of alkanes and alkenes.
Differentiation
- Resource Utilization: Converts otherwise wasted hydrocarbons into marketable products
- Sustainability: Reduces methane and carbon dioxide emissions by converting light hydrocarbons into valuable chemicals
- Efficiency: Operates under mild conditions with high selectivity producing high yields
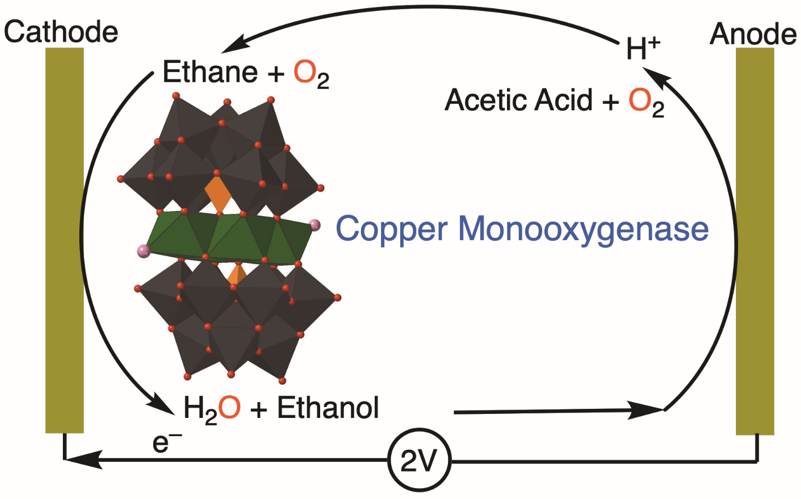
Electrocatalysis of ethane to acetic acid using copper–tungsten catalyst in a membrane-less electrolyzer
Development Stage
First proof of concept was demonstrated.
References
Patent Status:
European Patent Office Published: Publication Number: 4168609
Contact for more information

Dr. Vered Pardo Yissar
Senior Director of Business Development, Exact Sciences

