Salbutamol, also known as Ventolin, is a generic drug used for asthma treatments. It is one of the most common medications in the world as millions are using it daily. The mass production of Salbutamol usually consumes expensive reagents and generates a large amount of chemical waste. As Salbutamol's annual sales are in the multitone scale, any improvement in its synthesis is significant.
Prof. David Milstein and his coworkers developed a specially designed manganese-based catalyst that catalyzes a crucial synthetic step in the synthesis of Salbutamol. This inexpensive catalyst prevents the use of the costly Vitride reagent, which generates large amounts of chemical waste.
Over 3 percent of the world population, about 260 million people, are suffering from asthma. The most common way to handle this long-term disease is by inhaling Salbutamol which immediately opens up the airways in the lungs. Since 2020 Salbutamol has become a generic drug that any pharmaceutical company can sell, but due to its complex molecular structure, its synthesis remains challenging. Although it is sold on a multitone scale, its synthesis still involves expensive reagents. It creates a large amount of toxic chemical waste, especially in the synthesis of Salbutamol intermediate, where the costly Vitride reagent is used in large amounts and generates large amounts of aluminum salts and sodium- salts waste.
Prof. Milstein and his team developed a superior process in which the Salbutamol intermediate is formed by catalytic hydrogenation of the same starting compound using newly developed manganese catalysts, thus avoiding the use of the Vitride reagent
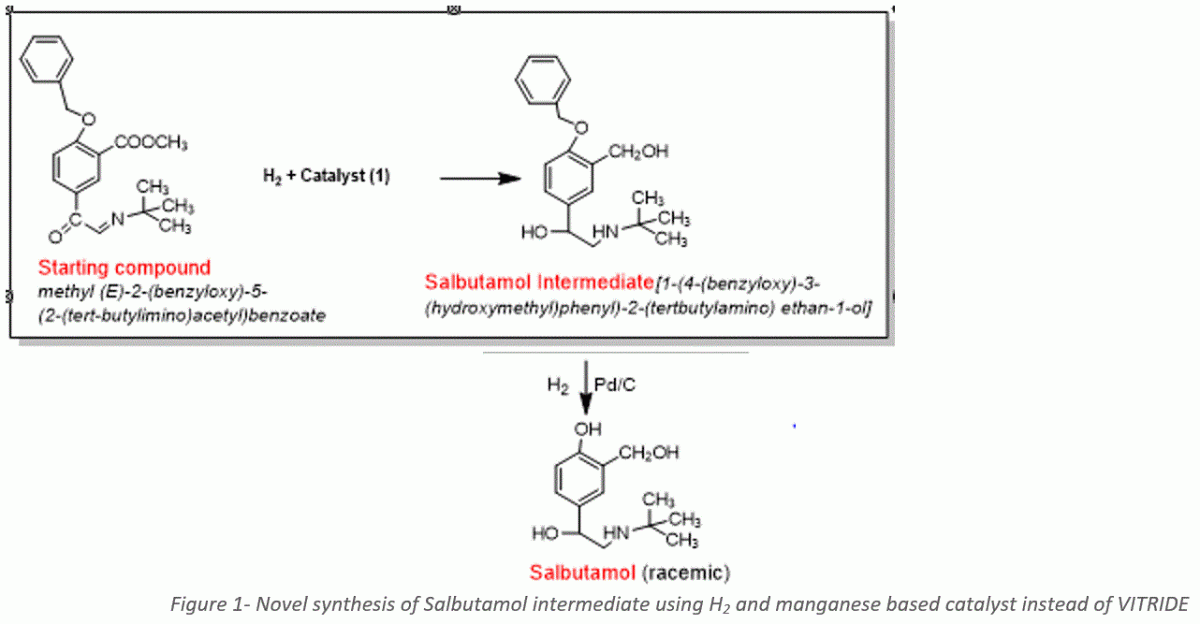
The synthesis of Salbutamol intermediate involves the reduction of three functional groups: ester, ketone, and imine. In the original procedure, Vitride (sodium bis(2-methoxyethoxy)aluminium hydride) must be used in excess to ensure the reduction of all three groups. The reduction process generates large amounts of aluminum-and sodium salts waste causing difficulties in purification and leaving much chemical waste. In the proposed reaction, the reduction source is hydrogen gas which is cheap and easy to remove and reuse after the reaction is completed, and the hydrogenation catalyst, based on low-cost, earth-abundant manganese, is cheap and used in minute amounts (2 mol%). The only byproduct is methanol. and no waste is produced. Salbutamol intermediate is easily purified. The procedure is very efficient, with up to 99% yield, and the catalyst is based on the low-cost metal in only a 0.02 molar ratio.
Applications
- Salbutamol synthesis
Advantages
- Almost no chemical waste
- Inexpensive metal-based catalyst
- Up to 99% yield
The team developed a series of metal-based catalysts, established and calibrated the chemical procedure for salbutamol synthesis using these catalysts, and determined the yields obtained under the different conditions of temperature, pressure, and reaction time.
The Salbutamol annual market is estimated to be 51 billion USD. The proposed synthesis is expected to significantly lower the production costs of this crucial medication in terms of both reagents and chemical waste handling.


