Nanostructured silica is widely used in catalysis, encapsulation, drug delivery, bioimaging, biosensing, energy storage, and more. Current synthesis methods rely on harsh chemicals, are costly, and environmentally unfriendly. This technology offers a biomimetic method for synthesizing nanostructured silica via polyanion–polycation phase separation in water. By adjusting reaction conditions, it enables precise control over morphology, size, and porosity, eliminating harsh chemicals and solvents for a more sustainable, efficient process.
- Catalysis
- Drug delivery
- Biosensing
- Bioimaging
- Food industry
- Paints
- Substances encapsulation
- Scaffold for tissue engineering
- Inexpensive and Highly stable
- Tailored morphology, sizes and porosity
- Biocompatible
- Environmentally Friendly
- Easily modified surfaces
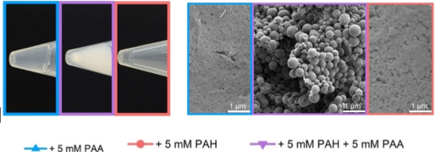
Silicification of Si(OH)4 in aqueous solution with polyanion (blue), polycation (red), and both polyanion and polycation (purple)
Varied morphologies of nanostructured silica were synthesized consistently in small scale from Si(OH)4. Applicative uses are still in development and need further research.

Dr. Vered Pardo Yissar

