Kidney stones affect millions worldwide, with up to 15% of the population experiencing them during their lifetime. Obstructing stones often require insertion of a temporary ureteral stent to maintain urine flow. Current double-J “pigtail” stents are associated with substantial discomfort, as over 80% of patients report pain affecting daily activities, and do not promote stone clearance. The YotiCurl stent is a novel, single-use ureteral stent that is designed to alleviate patient discomfort, is easily inserted and removed, and may actively encourage stone mobilization and migration to the bladder. Proof-of-concept testing in a pig model showed safe and effective use.
- Relief of ureteral obstruction caused by kidney stones
- Pre- and post-surgical management of urinary tract procedures
- Treatment of benign and malignant ureteral obstructions
- Patient comfort: Designed to significantly reduce pain compared to double-J stents
- Ease of use: Simple insertion and removal using current clinical practice
- Stone clearance: Design may facilitate spontaneous stone mobilization
- Compatibility: Single-use, compatible with existing stent manufacturing
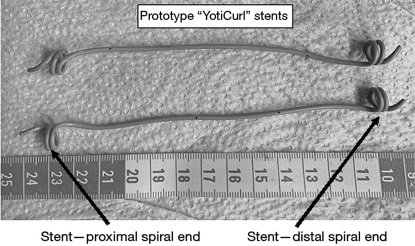
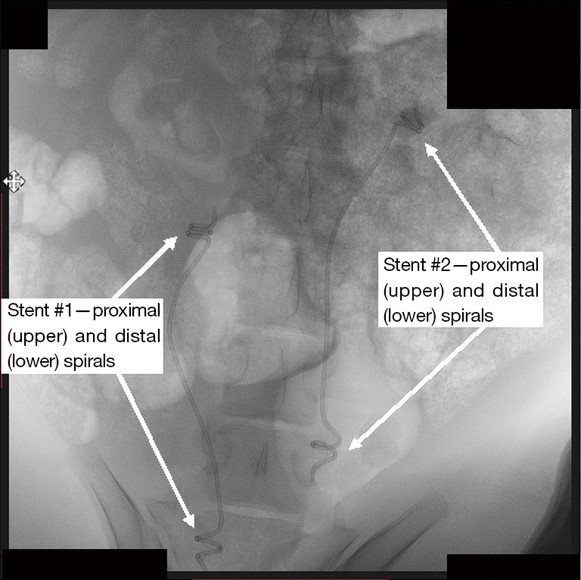
Prototype “YotiCurl” stents emplaced in vivo in a pig model
Prototype stents have been synthesized and successfully tested in a live pig model, demonstrating safe emplacement, full functionality, no stent migration, absence of inflammation, and favorable histological outcomes.
Epidemiological data reveal that the prevalence of stone disease is increasing, indicating that demand for ureteral stents is likely to increase. It is estimated that kidney stones affect 1 in 11 people in the USA. The market for ureteral stents is driven primarily by the increasing numbers of cases of kidney stones, presumably owing to various factors attributed to Western diets – high usage of animal protein, salt, and vitamin supplements – as well as a sedentary lifestyle and global climate warming.

Dr. Vered Pardo Yissar

