5728
Overview
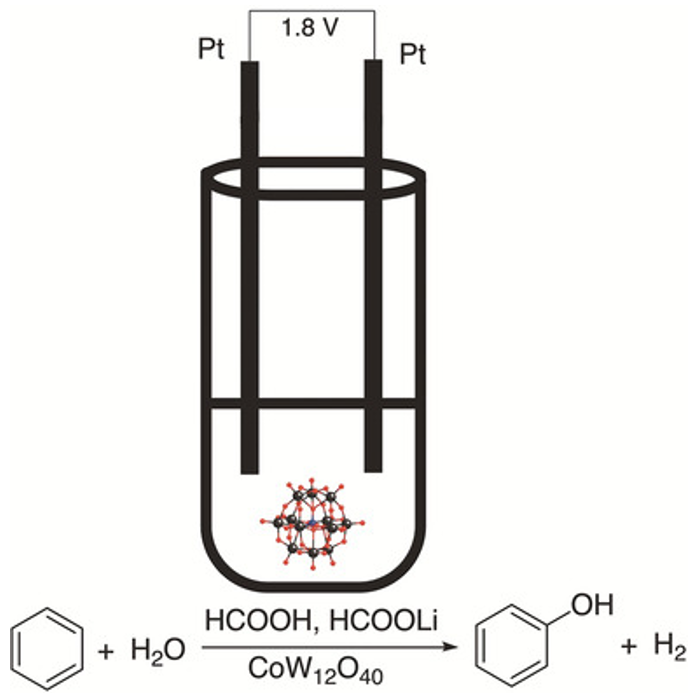
A sustainable method for phenol production using electrocatalytic oxidation of benzene.
Applications
- Production of phenol from benzene
- Production of acetaldehyde from ethylene
- Production of malonic and/or pyruvic acids from acrylic acid
- Production of various alcohols from aliphatic and aromatic hydrocarbons
Differentiation
- Safe – process in low Temp.
- Highly Selective (no acetone as co-product)
- Cost-Effective
- Simple and fast process
Development Stage
A proof of concept was demonstrated on a lab scale.
Non-optimized reaction conditions: Faradaic efficiency of 75% and 35% yield.
Patent Status:
USA Granted: 11,174,561 USA Granted: 11,795,553
Contact for more information

Dr. Vered Pardo Yissar
Senior Director of Business Development, Exact Sciences

