Current methods for synthesizing primary amines require high energy input, toxic reagents, and result in undesired byproducts. This technology offers a direct, single-step catalytic process using alcohols and ammonia under mild conditions. Catalyzed by a novel Pincer-type Ruthenium complexes, it enables efficient and selective production of primary amines while minimizing waste and reducing production costs.
Industrial synthesis of primary amines for pharmaceuticals, agrochemicals, polymer and surfactant production, and fine and specialty chemicals
- Single-step reaction with high selectivity for primary amines
- Operates under mild conditions (~130 °C, 1 atm)
- Utilizes water or no solvent at all
- No toxic reagents or byproducts
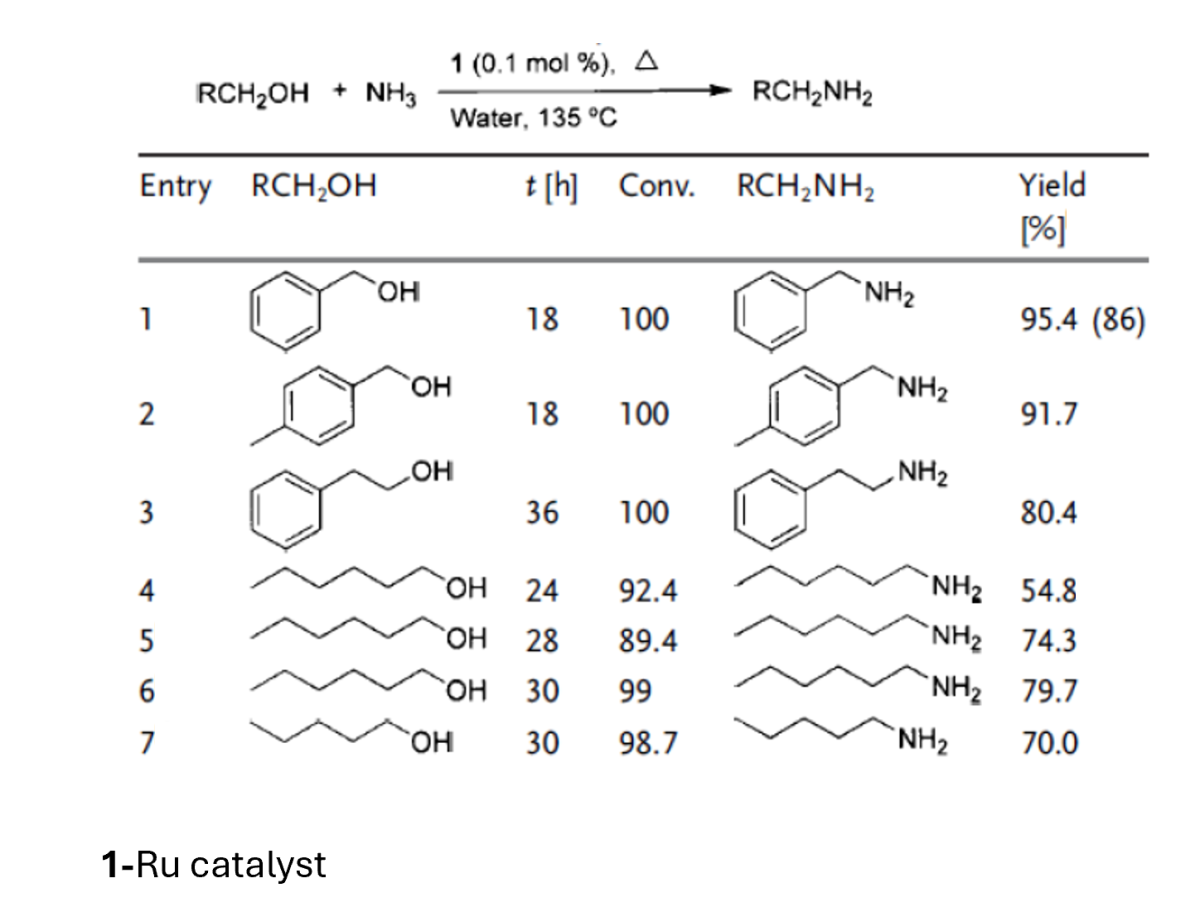
Proof of concept demonstrated for a range of alcohols, including aromatic and aliphatic compounds.

Dr. Vered Pardo Yissar

