Immunotherapy can induce durable tumor regression in patients with metastatic cancer, largely driven by the immune system’s recognition of tumor-specific neoantigens. We developed a robust, high-throughput platform for the identification and biological validation of HLA class I and II recurrent immunogenic neoantigens and their corresponding TCRs.
Leveraging this platform, we successfully identified and validated a suite of TCRs targeting clinically relevant neoantigens—including those derived from hotspot mutations in NRAS, PIK3CA KRAS, HRAS, and the androgen receptor—enabling the development of TCR-based therapies for a broad range of solid tumors.
- Discovery and validation of TCRs against common immunogenic neoantigens
- Off-the-shelf TCR cell therapy for cancer patients
- Extension to microbial, viral, or autoimmune diseases
- Neoantigen mRNA-based vaccines
- Discovery of common and highly immunogenic neoantigens
- Robust biological validation of TCRs
- Identification of both HLA class I and class II neoantigens
- TCRs with a higher probability of clinical success
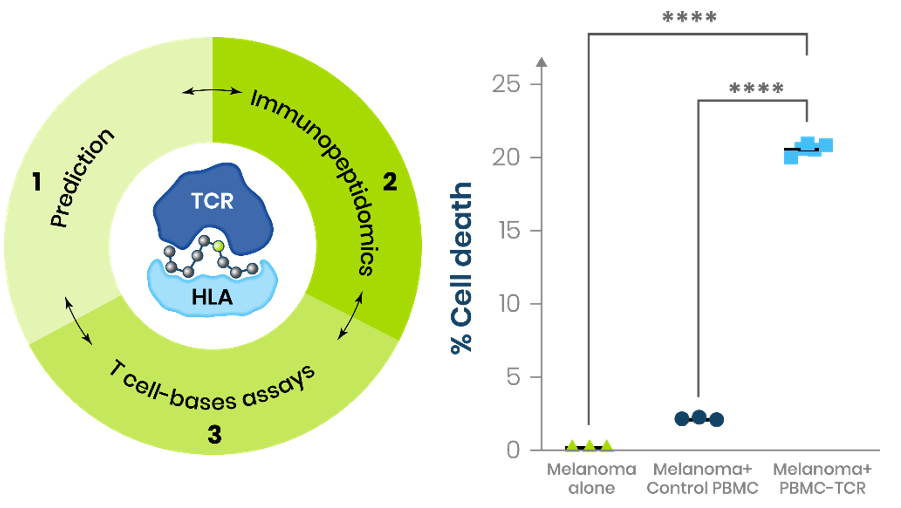
Engineered T cells expressing NRAS-specific TCR effectively eliminate neoantigen-expressing melanoma cells
Pipeline of TCRs ready for clinical trials targeting NRAS (Q61K), NRAS (Q61R), KRAS (Q61K), KRAS (G12V), KRAS (G12C), HRAS (Q61K), Androgen Receptor (H875Y). Additional TCRs are in discovery and validation stages


