Text 4
Inteins are protein domains that auto-catalyze protein-splicing of their flanking regions with a peptide bond. In protein trans-splicing, two parts of a split-intein can be used to ligate or cyclize polypeptides in a traceless manner. The reaction is robust, can be performed in-vivo or in-vitro, using biologically or chemically synthesized peptides or proteins. The sequence of only 2-3 flanking amino acids is constrained. As shown in the figure below, the N and C terminal intein fragments (IntN & IntC) associate and fold into the active domain, thereby linking the flanking sequences with a peptide bond. All previously known split inteins only function in reducing conditions due to their use of one or two catalytic cysteines. In this invention, novel cysteine-less split inteins are capable of robust trans-splicing at ambient temperatures and without the requirement of any chemical reducing or denaturation steps. This allows the preservation of disulfide bonds within the target protein. The use of this split intein was demonstrated for a full-length IgG, an Fc fragment of an IgG antibody, and two nanobodies as representatives of therapeutically relevant proteins. The reactions are high yielding (> 90%) at low to medium micromolar concentrations.
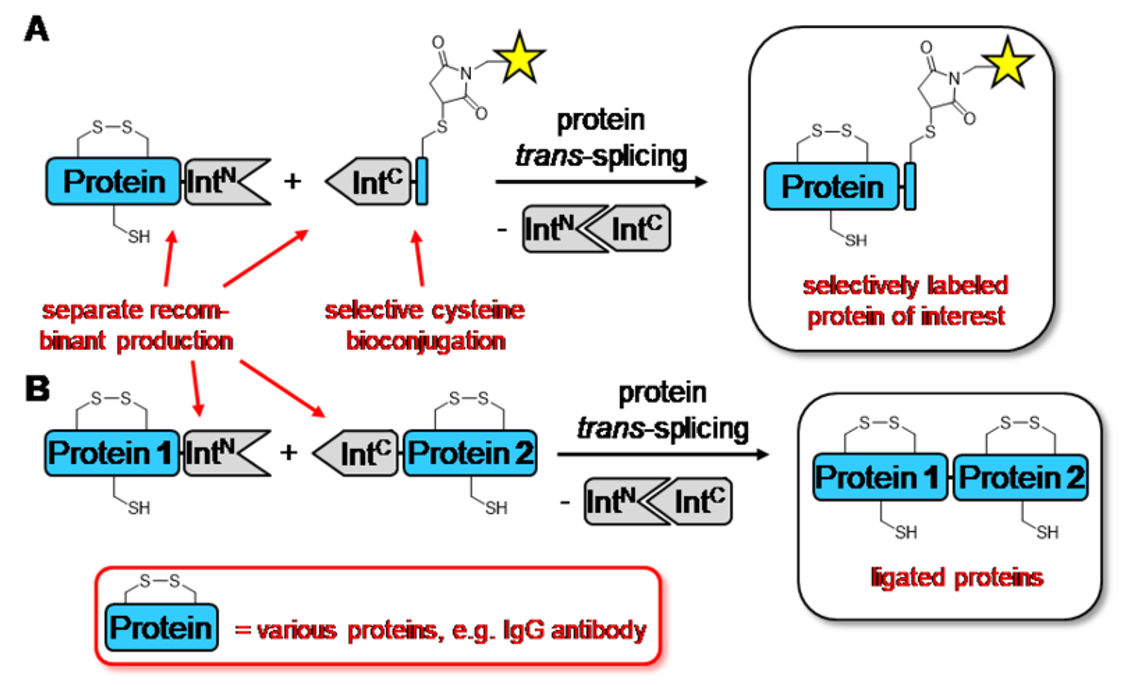
Figure 1 – Inteins mechanism of action
