Pathogenic fungi that cause postharvest decay of fruits and vegetables result in massive yield loss. The gray-mold disease affects various plant species, and the current treatments of non-specific fungicides are not eco-friendly and may impact human health. Therefore, there is a need for eco-friendly approaches to prevent postharvest disease effectively. The teams of Prof. Robert Fluhr and Prof. Noam Alkan of the Volcani Institute have developed a fungal-specific double-stranded RNA (dsRNA) that, upon external application on plants, specifically inhibits fungal growth and prevents crop decay. Incorporating the dsRNA in nano-clay particles protects the dsRNA from degradation, thus prolonging the dsRNA shelf life, and serves as a slow release mechanism to improve effectiveness.
More than 40% of the global yield of fruits and vegetables is lost due to postharvest decay caused by pathogenic fungi. Botrytis cinerea, which causes gray mold disease, is one of the most common necrotrophic fungal pathogens, affecting a wide range of plant species. B. cinerea usually infects the plants in the field but remains latent until harvest. Fruits lose their innate resistance during ripening and become susceptible to dormant fungal pathogens, which cause gray mold once activated1,2. Currently, fungicides are the most effective treatment to prevent postharvest diseases. However, due to their lack of specificity, fungicides may harm beneficial micro-organisms, impact human health, and result in the emergence of resistance to fungicides. Therefore, there is a need to develop eco-friendly approaches to control fungal pathogens and postharvest diseases.
Prof. Robert Fluhr and Prof. Noam Alkan of the Volcani Institute designed fungal-specific dsRNA that inhibits fungal growth upon external application on plants, thus preventing fruit decay3. They further incorporated the dsRNA in a layered-double hydroxide (LDH) nano-clays.
RNA interference (RNAi) process is based on cellular recognition of double-stranded RNA (dsRNA) molecules that leads to sequence-specific degradation or interference of translation of a target messenger RNA. Previous work demonstrated that external application of dsRNA on plant parts such as leaves, branches, or fruit, can effectively reduce fungal growth4. This technique, known as spray-induced gene silencing (SIGS), has clear advantages and offers plant protection. Prof. Fluhr and Prof. Alkan designed dsRNA that targets the fungal ergosterol biosynthesis pathway. Ergosterol is a C28 sterol found mainly in fungal cell membranes. It is essential for fungal growth and survival, making it a favorable target for various existing fungicides5. team demonstrated that the dsRNA penetrates B. cinerea, reduces germination and hyphal growth in vitro and in vivo, and inhibits gray mold development on various fruits (including bell pepper cherry, strawberry, mango, and grape. Figure 1a). They also showed that SIGS combined with the commercially used fungicides (i.e., prochloraz or fludioxonil), worked synergistically and could significantly reduce the amount of fungicide needed to inhibit fungal growth (Figure 1b). To protect the dsRNA from washing, RNase degradation, and U.V. exposure, the scientists incorporated it in layered-double hydroxide (LDH) nano-clays. Increased levels of CO2 and humidity increase LDH degradation, thus slowly releasing the dsRNA from the complex allowing its uptake by the fungi. Treatment with the LDH-dsRNA complex reduced B. cinerea germination and growth (Figure 2a) and reduced natural decay development during storage (b).
- Antifungal treatment to increase the shelf-life of various crops and harvested fruits
- Eco-friendly and highly specific
The team designed the dsRNA and tested them in vitro and in vivo, as well as their combination with commercially available fungicides.
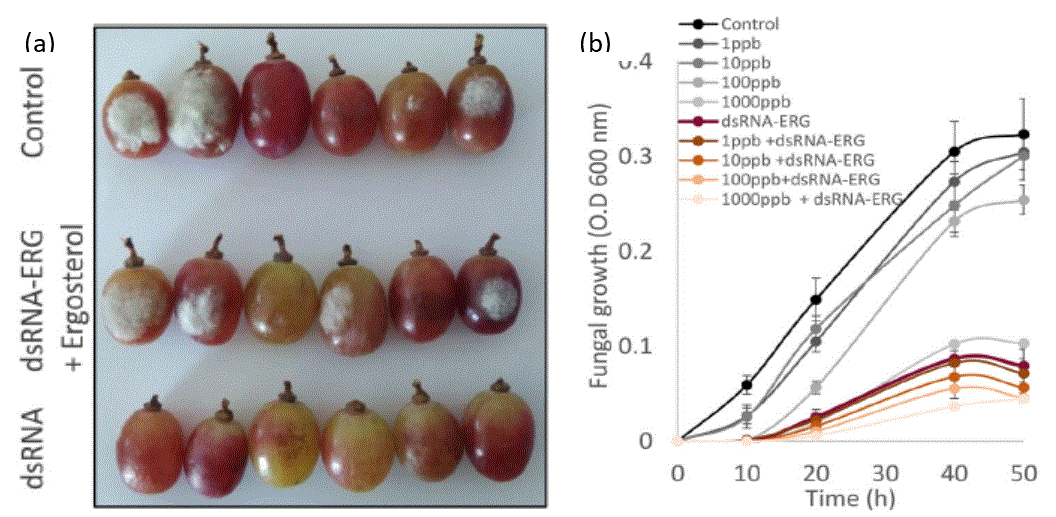
Figure 1- (a) Representative pictures of grapes treated with water (control), or dsRNA-ERG or dsRNA-ERG and ergosterol, following spray inoculation with B. cinerea were taken four days post-infection (dpi). (b) B. cinerea conidia were incubated at room temperature in standard media (control) or supplemented with various concentrations of Prochloraz, with or without dsRNA-ERG. O.D measurements at 600 nm were taken every hour. The presented data are mean and standard errors.
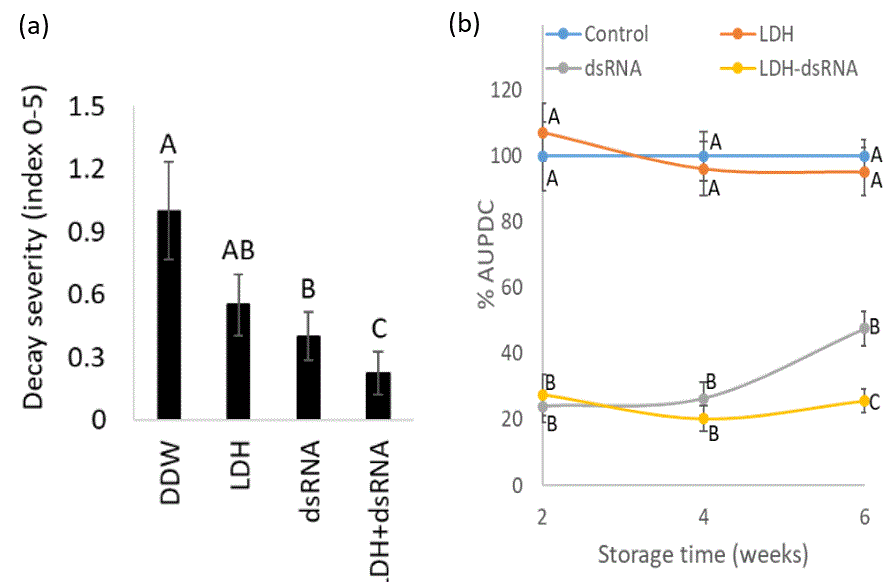
Figure 2 – (a) LDH-dsRNA complex reduces fruit decay caused by B. cinerea inoculation: After causing micro-injuries to the grape cuticles, spray treatment was applied with water (control), dsRNA, LDH, or LDH-dsRNA complex. The fruits were then sprayed with B. cinerea conidia and stored at 22°C. The graph shows the decay severity (index 0-5) four days post-inoculation. (b) LDH-dsRNA prolongs fruits' shelf life: Grapes were treated with water (control), LDH, dsRNA, or LDH-dsRNA and stored at 0°C. After 2, 4, and 6 weeks, fruits peel were micro-injured, followed by spray inoculation by conidia of B. cinerea. Decay severity was recorded on days 3-5, and the area under the disease progress curve (AUDPC) was calculated.
- Abiad MG, Meho LI. Food loss and food waste research in the Arab world: a systematic review. Food Secur. 2018;10(2):311-322. doi:10.1007/s12571-018-0782-7
- Flanagan K, Robertson K, Hanson C. Reducing Food Loss and Waste: Setting a Global Action Agenda. World Resour Inst. Published online 2019. doi:10.46830/wrirpt.18.00130
- Duanis‐Assaf D, Galsurker O, Davydov O, et al. Double‐stranded RNA targeting fungal ergosterol biosynthesis pathway controls Botrytis cinerea and postharvest gray mould. Plant Biotechnol J. Published online November 18, 2021:pbi.13708. doi:10.1111/pbi.13708
- Koch A, Biedenkopf D, Furch A, et al. An RNAi-Based Control of Fusarium graminearum Infections Through Spraying of Long dsRNAs Involves a Plant Passage and Is Controlled by the Fungal Silencing Machinery. Dinesh-Kumar SP, ed. PLOS Pathog. 2016;12(10):e1005901. doi:10.1371/journal.ppat.1005901
- Ahmad A, Khan A, Manzoor N, Khan LA. Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microb Pathog. 2010;48(1):35-41. doi:10.1016/j.micpath.2009.10.001

